
The coverage on this live blog has ended — for up-to-the-minute coverage on the coronavirus, visit CNBC's latest live blog.
The United States is set to see a substantial boost in Covid-19 vaccines shipped out and ready to administer in the coming weeks. Executives from Pfizer and Moderna told Congress on Tuesday the drugmakers are preparing to nearly double their current output. And on Wednesday, Food and Drug Administration staff endorsed Johnson & Johnson's one-shot vaccine, clearing the path for an emergency use authorization as soon as this week. A J&J executive said once the drug is authorized, the company would be ready to ship more than 20 million doses by the end of March.
Here are some of the biggest developments Wednesday:
- J&J vaccine clears a key hurdle on the path to an emergency use authorization
- Moderna to begin clinical trials of Covid booster shots for South Africa variant
- Drugmakers vow to ramp up vaccine production after sluggish start
- These are the most common side effects from J&J's one-shot vaccine
Get a weekly recap of the latest San Francisco Bay Area housing news. Sign up for NBC Bay Area’s Housing Deconstructed newsletter.
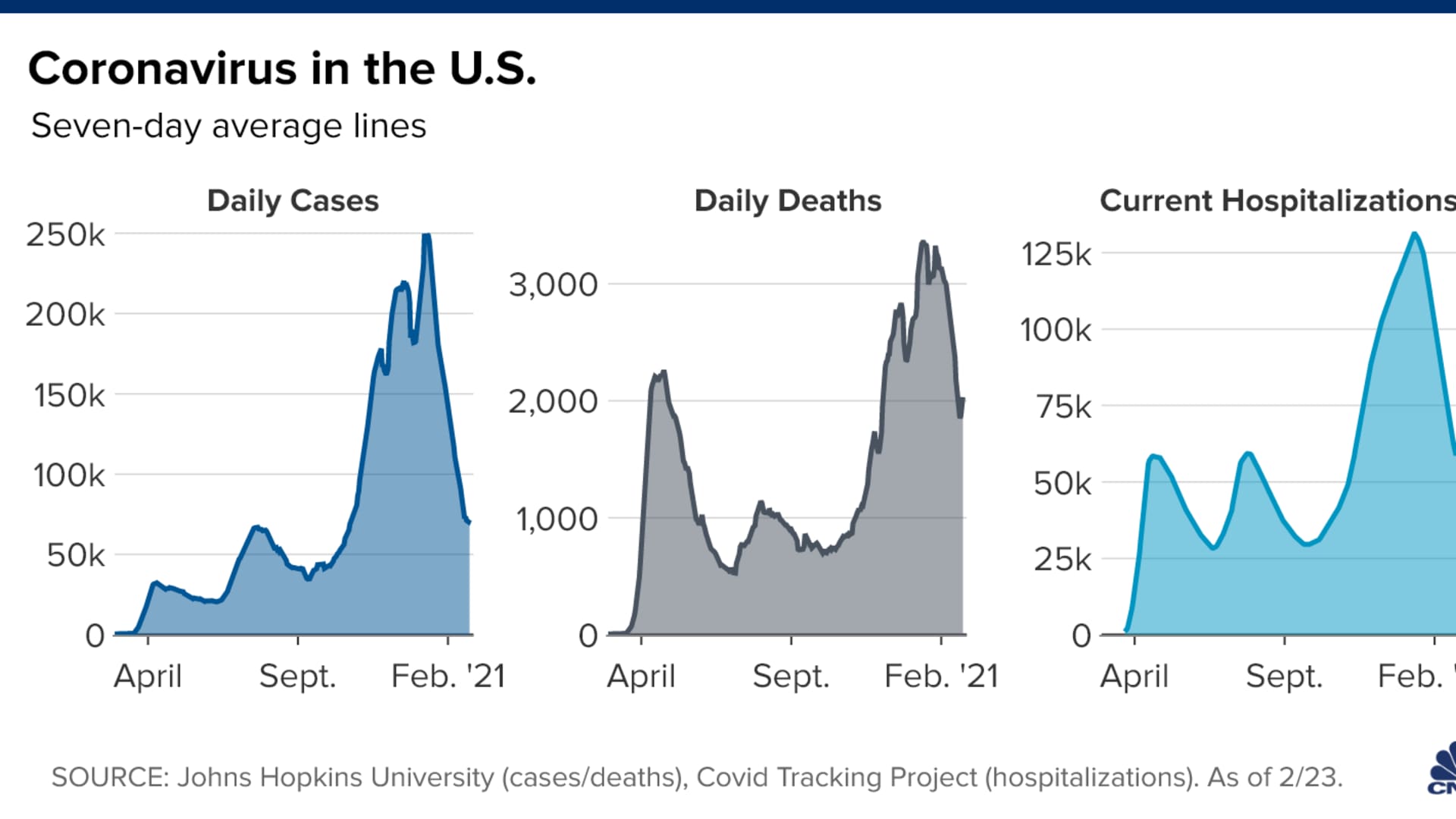
The U.S. is recording at least 71,500 new Covid-19 cases and at least 2,030 virus-related deaths each day, based on a seven-day average calculated by CNBC using Johns Hopkins University data.
The following data was compiled by Johns Hopkins University:
- Global cases: More than 112.4 million
- Global deaths: At least 2.49 million
- U.S. cases: More than 28.32 million
- U.S. deaths: At least 504,738
U.S. should expedite AstraZeneca vaccine authorization before new Covid variants spread, Dr. Peter Hotez says
Money Report
Dr. Peter Hotez warned that people in the U.S. should not become complacent because of dropping Covid cases, especially amid new reports of a new variant, B.1.526, spreading in New York.
"We're all high fiving ourselves because the numbers are going down, and I am saying that we are in the eye of the hurricane, and the next big wave is coming," said the co-director of the Center for Vaccine Development at Texas Children's Hospital.
Average daily cases of coronavirus in the U.S. have dropped about 57%, according to a CNBC analysis of Johns Hopkins data. Some states, however, are not seeing as sharp of a decline. Vermont is only down 22% in average daily cases, New York down about 45%, Oregon nearly 47%, and Florida is down 48% in daily average cases. Hotez singled Florida out for the prevalence of a highly transmissible Covid variant in the state that was first found in the U.K.
"The one state that really intrigues me, not necessarily in a good way, is Florida, because we are hearing that about 10% of the virus isolates coming out of Florida is that B.117 variant that came out of the United Kingdom," Hotez said.
—Emily Deciccio
CDC director says virus variants could ‘undermine all of our efforts’
The emerging, highly transmissible coronavirus variants "stand to reverse" the nation's control of the pandemic and could "undermine all of our efforts" against the disease if the virus is left to proliferate in different parts of the globe, Centers for Disease Control and Prevention Director Dr. Rochelle Walensky said.
Top health officials have warned in recent weeks that the emergence of the variants, particularly the B.1.1.7 strain that arose in the U.K., could reverse the current downward trajectory in infections in the U.S. and delay the nation's recovery from the pandemic. The problem isn't isolated to the U.S. As more people become infected, the more likely it is that problematic mutations will arise, experts warn.
"Even if you were not necessarily leaning towards wanting to be part of the global health effort, we need to because all of the efforts that we're doing, that we are moving forward here in this nation, could be potentially undermined in a heartbeat" from the variants, Walensky told the National Academy of Medicine and the American Public Health Association.
—Noah Higgins-Dunn
House plans to pass $1.9 trillion Covid relief bill on Friday
The House plans to pass its $1.9 trillion coronavirus relief package as early as Friday.
The Senate will then consider the bill and aims to approve it before March 14. Programs buoying millions of unemployed Americans are set to expire next month.
Democrats are expected to pass the plan without any Republican votes through the budget reconciliation process.
One major issue in the process looms. The Senate parliamentarian will determine as soon as this week whether the chamber can put a $15 per hour minimum wage — included in the House bill — into the legislation under the strict rules governing reconciliation.
— Jacob Pramuk
Moderna to begin clinical trials of Covid booster shots for South Africa variant
Moderna said it has shipped to the National Institutes of Health doses of a new Covid-19 vaccine designed to provide better protection against the highly contagious coronavirus variant spreading in South Africa.
The vaccine, called mRNA-1273.35, is ready to be tested in an early-stage clinical trial to determine if it can be used as a booster shot against the South African strain, also known as B.1.351, the company said.
U.S. health officials are growing concerned about new, emerging variants of the virus, particularly the B.1.351 strain. In recent weeks, White House Chief Medical Advisor Dr. Anthony Fauci has pushed Americans to get vaccinated as quickly as possible before potentially new and even more dangerous variants of the virus emerge.
Moderna has found its current two-dose regimen generates a weaker immune response against the strain from South Africa, though the company said antibodies in patients remain above levels that are expected to be protective against the virus.
–Berkeley Lovelace Jr.
President Biden extends national emergency
President Joe Biden extended the declaration of a national emergency in response to the Covid-19 pandemic beyond its March expiration date. The original declaration was made on March 13, 2020 and would have expired after one year.
"The COVID-19 pandemic continues to cause significant risk to the public health and safety of the Nation," the declaration says. "It is essential to continue to combat and respond to COVID-19 with the full capacity and capability of the Federal Government."
–Rich Mendez
Retail sales expected to jump this year as more Americans get vaccinated

Retail sales are expected to jump this year as Covid vaccines usher in an economic rebound, with the National Retail Federation estimating $4.33 trillion in sales, CNBC's Lauren Thomas reports.
The industry group reported a 6.7% growth in retail sales last year on the back of a 22% surge in online retail. With more Americans receiving vaccines and lockdowns lifting, the group says the surge in retail sales will continue this year and could fall somewhere between 6.5% and 8.2%.
"Our principal assumption is that ... the vaccination will be effective and permits accelerated growth during the mid-year," NRF chief economist Jack Kleinhenz said in a statement. "The economy is expected to see its fastest growth in over two decades."
–Rich Mendez
Fauci says data suggests 'long' Covid symptoms can last up to 9 months

New data suggests that people with Covid-19 can continue to suffer from symptoms for months after the initial infection, White House Chief Medical Advisor Dr. Anthony Fauci told reporters.
Researchers at the University of Washington recently found that 30% of patients reported symptoms for as long as nine months, he said. Symptoms of "long Covid," which researchers are now calling Post-Acute Sequelae of Covid-19, or PASC, can develop "well after" infection, and severity can range from mild to "incapacitating," he said.
"The magnitude of the problem is not fully known," Fauci said, adding PASC was also reported in people who did not require hospitalizations or people who had symptoms that were not part of their initial infection.
–Berkeley Lovelace Jr.
U.S. ready to roll out J&J vaccine 'without delay,' top Biden health official says

The U.S. is ready to deliver Johnson & Johnson's one-shot Covid-19 vaccine as early as next week if the shot is granted emergency authorization from the U.S. Food and Drug Administration, the Biden administration's Covid-19 response team said.
"The governors are carefully planning their efforts and are getting ready for the possible new vaccine," Jeff Zients, President Joe Biden's Covid czar, said at a news briefing. "If authorized, we're ready to roll out this vaccine without delay."
The administration plans on allocating between 3 million to 4 million doses of J&J's vaccine next week, pending authorization. The company said it aims to provide the country with 20 million doses by the end of March and 100 million doses by the end of June, Zients said.
—Noah Higgins-Dunn
More than 150 CEOs call on Congress to pass Biden's Covid stimulus package
More than 150 New York CEOs called on Congress in a letter to pass President Joe Biden's $1.9 trillion Covid relief bill.
The letter's signatories include Goldman Sachs' David Solomon, BlackRock's Larry Fink, Deutsche Bank Americas' Christiana Riley and Blackstone's Steve Schwarzman, who previously backed former President Donald Trump.
A day prior, the Business Roundtable, an association of chief executives at the country's largest companies, also wrote a letter supporting the passage of Covid relief.
CNBC reported earlier in February that the White House reached out to business executives to rally support for Biden's American Rescue Plan.
—Hannah Miao
These are the most common side effects from J&J’s one-shot vaccine
Headaches, fatigue and muscle pain were some of the most common side effects among people who received Johnson & Johnson's one-shot vaccine in clinical trials, according to a new report from the U.S. Food and Drug Administration.
Nearly half of vaccine recipients participating in J&J's clinical trials reported injection site pain following inoculation, compared with roughly 17% in the placebo group, according to the FDA. Almost 40% of people who received the drug reported experiencing a headache and just over 38% reported feeling fatigued. Most people were able to shake off the side effects within a couple of days following their shot, the report said.
Medical experts advised that side effects following vaccination are common, and they typically are indications that it's helping build protection against the disease.
—Noah Higgins-Dunn
VP Harris' press pool sent home after member tests positive for Covid

The group of journalists covering Vice President Kamala Harris was sent home Wednesday morning after one of its members tested positive for Covid, the White House said.
The unidentified person tested positive for the virus during routine screening and had no contact with Harris or other White House staff, according to a statement from the office of President Joe Biden.
"As soon as we were notified, we disbanded the Vice President's pool and sent them home. They will be tested again tomorrow," the statement said.
"Out of an abundance of caution, we made arrangements for the briefing room to be cleaned," Biden's office said, adding that the White House has begun the process of contact tracing.
Harris is scheduled to join Biden today to receive the daily intelligence brief in the Oval Office and later to have lunch with the president. Biden and Harris will then meet with labor leaders at the White House to discuss the president's infrastructure goals and the Covid relief bill. All of those events are set to be held without reporters present, though the press pool may hear from Biden at the top of the meeting with labor leaders.
—Kevin Breuninger
Drugmakers vow to ramp up vaccine production after sluggish start
Drugmakers are significantly ramping up Covid-19 vaccine production after working out manufacturing issues that initially delayed the first shots, executives testified before Congress Tuesday.
Rep. Diana DeGette, D-Colo., pressed Pfizer and Moderna executives on why they missed early U.S. delivery targets of their vaccines, saying that "the amount of supply has fallen short of expectations."
"We did initially experience some problems with the initial ramp-up of our vaccine," Pfizer Chief Business Officer John Young testified at the hearing. "In common with other panelists here, we've been in the process of developing a manufacturing process for a vaccine product that we've never made before."
Moderna President Dr. Stephen Hoge defended the company's progress, noting that it just narrowly missed its goal of delivering 20 million doses by the end of last year. The company, he said, delivered 17.8 million doses by Dec. 31.
"We ultimately had never, when we were trying to make those estimates, manufactured doses at this scale," Hoge said at the hearing. "We had a lot to learn along the way, and many of the challenges that we ran into were the normal sort of training experiences as you train people to operate a complicated process."
—Will Feuer
France hopes to avoid third lockdown with extra local restrictions
CNBC's Charlotte Reed reports on fresh local restrictions being introduced in France as new Covid-19 cases remain on a high plateau and the pressure grows on intensive care units.
J&J vaccine clears a key hurdle on the path to an emergency use authorization
Johnson & Johnson's one-shot Covid vaccine cleared a key hurdle on the path to an emergency use authorization, earning an endorsement by staff at the Food and Drug Administration, CNBC's Berkeley Lovelace Jr. reports.
The staff report comes ahead of an FDA advisory panel meeting on Friday and could mean the green light for a third U.S. vaccine as soon as this week.
The vaccine was found to be 66% effective in protecting against the virus, though less so in regions where virus variants are taking hold. Still, the drug prevented 100% of virus-related hospitalizations and deaths.
—Sara Salinas
Lowe's warns of likely drop in home improvement demand in coming months

As more Americans feel safe going out to dinner or getting away for the weekend again, Lowe's said it expects a drop in demand for cans of paint and other supplies for do-it-yourself projects.
The retailer topped analysts' expectations for the fiscal fourth quarter, as same-store sales grew by 28.1%. The company said it continued to benefit from people investing in their homes during the pandemic.
Lowe's warned, however, that strong demand for home improvement will moderate as more people get Covid-19 vaccines and spend less time at home. The company reiterated a forecast that it gave at an investor day in December, saying demand will likely decline by 5% to 7% — even in a robust market.
—Melissa Repko
CVS to start offering vaccines in six more states

CVS will start offering Covid vaccine shots in six more states on Thursday, expanding the roster to a total of 17 states. The latest additions are Alabama, Arizona, Florida, Louisiana, Ohio and Pennsylvania.
Drugstores are likely to be a key player in getting doses into the arms of the general population.
The drugstore chain was already offering the vaccine at pharmacies in California, Connecticut, Hawaii, Maryland, Massachusetts, New Jersey, New York, Rhode Island, South Carolina, Texas and Virginia.
—Sara Salinas
Read CNBC's previous live coverage here:
Covid updates: Biden ups weekly vaccine shipments; 'variants of concern' have U.S. officials on edge






